Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 MoO
MoO , in severe accident conditions, 1; Partitioning of Cs and Mo among gaseous species
, in severe accident conditions, 1; Partitioning of Cs and Mo among gaseous speciesDo, Thi Mai Dung*; Sujatanond, S.*; Ogawa, Toru
Journal of Nuclear Science and Technology, 55(3), p.348 - 355, 2018/03
Times Cited Count:6 Percentile:52.79(Nuclear Science & Technology)In order to better understand the behavior of cesium in the severe accident of the LWR, the high-temperature chemistry of Cs MoO
MoO in H
in H O+H
O+H gas was studied. The pseudo-binary system, Cs
gas was studied. The pseudo-binary system, Cs MoO
MoO -MoO
-MoO , was thermochemically modeled with Redlich-Kister formulation to form a basis to analyze the high-temperature behavior of Cs
, was thermochemically modeled with Redlich-Kister formulation to form a basis to analyze the high-temperature behavior of Cs MoO
MoO . The model prediction was compared with the thermogravimetric measurements of Cs
. The model prediction was compared with the thermogravimetric measurements of Cs MoO
MoO in dry and humid argon, which revealed that the mass-loss rate was enhanced in the humid atmosphere. Thermochemical model was further applied to predict the partitioning of cesium and molybdenum among gaseous species in the BWR core degradation condition typical of Short-Term Station Blackout.
in dry and humid argon, which revealed that the mass-loss rate was enhanced in the humid atmosphere. Thermochemical model was further applied to predict the partitioning of cesium and molybdenum among gaseous species in the BWR core degradation condition typical of Short-Term Station Blackout.
Nakajima, Kunihisa
Mass Spectrometry (Internet), 5(2), p.S0055_1 - S0055_6, 2016/12
Though equilibrium vapor pressures are utilized to determine thermodynamic properties of not only gaseous species but also condensed phases, the obtained data often disagree by a factor of 100 and more. A new data analysis method is proposed using the so-called second and third law procedures to improve accuracy of vapor pressure measurements. It was found from examination of vapor pressures of cesium metaborate and silver that the analysis of the difference between the second and third law values can result in determination of an optimal data set. Since the new thermodynamic method does not require special techniques and or experiences in dealing with measured data, it is reliable and versatile to improve the accuracy of vapor pressure evaluation.
Takai, Toshihide; Nakajima, Kunihisa; Furukawa, Tomohiro
JAEA-Technology 2015-002, 20 Pages, 2015/03
To improve the evaluation technique of source term, the measurement technique of the equilibrium vapor pressure using a high temperature mass spectrometer is required to expand the thermodynamic database of the simulated FPs. Existing test apparatus was adapted for this purpose. A mass spectrometer capable of measuring a wide mass number range and glove box for handling simulated FPs were installed for analyzing heavy FPs and preventing deterioration of simulated FPs in an air atmosphere, respectively. Function verification using standard sample and precision investigation using simulated FP sample were carried out. The oxygen dissociation pressure and standard enthalpy of formation of RuO (s) were evaluated, and it was confirmed these evaluated values were agreed with the calculated value from existing thermodynamic data and evaluation value written in the literature. Consequently, it was proven that high precision thermodynamic data was able to obtain by using this apparatus.
(s) were evaluated, and it was confirmed these evaluated values were agreed with the calculated value from existing thermodynamic data and evaluation value written in the literature. Consequently, it was proven that high precision thermodynamic data was able to obtain by using this apparatus.
Ohno, Shuji*; Miyahara, Shinya*; Kurata, Yuji
Journal of Nuclear Science and Technology, 42(7), p.593 - 599, 2005/07
no abstracts in English
Kato, Tetsuya*; Iizuka, Masatoshi*; Inoue, Tadashi*; Iwai, Takashi; Arai, Yasuo
Journal of Nuclear Materials, 340(2-3), p.259 - 265, 2005/04
Times Cited Count:23 Percentile:81.17(Materials Science, Multidisciplinary)no abstracts in English
Ohno, Shuji*; Miyahara, Shinya*; Kurata, Yuji
Proceedings of 12th International Conference on Nuclear Engineering (ICONE-12) (CD-ROM), 6 Pages, 2004/00
no abstracts in English
Koshizuka, Seiichi*; Ikeda, Hirokazu*; Liu, J.*; Oka, Yoshiaki*
JAERI-Tech 2002-013, 60 Pages, 2002/03
no abstracts in English
*; J.Huang*; *; *; *; Yamaguchi, Kenji*; Sugimoto, Jun
JAERI-Tech 98-003, 32 Pages, 1998/02
no abstracts in English
Nishihara, Tetsuo; Hada, Kazuhiko; Shiozawa, Shusaku
JAERI-Research 97-022, 110 Pages, 1997/03
no abstracts in English
; Abe, Yutaka*; *; *; Yamano, N.; Sugimoto, Jun
JAERI-Research 96-032, 152 Pages, 1996/06
no abstracts in English
Kunugi, Tomoaki; *
Fusion Engineering and Design, 28, p.162 - 169, 1995/00
Times Cited Count:1 Percentile:17.53(Nuclear Science & Technology)no abstracts in English
Ogawa, Toru; Omichi, Toshihiko; Maeda, Atsushi; Arai, Yasuo;
Journal of Alloys and Compounds, 224, p.55 - 59, 1995/00
Times Cited Count:23 Percentile:78.26(Chemistry, Physical)no abstracts in English



 U
U


 O
O

 solid solution
solid solutionUgajin, Mitsuhiro; ; Shiba, Koreyuki
Journal of Nuclear Materials, 116(2-3), p.172 - 177, 1983/00
Times Cited Count:15 Percentile:81.67(Materials Science, Multidisciplinary)no abstracts in English
Shimizu, Saburo; ; Nakajima, Hayato; Ikezoe, Yasumasa;
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 50(11), p.904 - 908, 1982/00
no abstracts in English
;
JAERI-M 9306, 40 Pages, 1981/02
no abstracts in English
JAERI-M 7583, 39 Pages, 1978/03
no abstracts in English

Nakajima, Kunihisa
no journal, ,
Results on the thermodynamic study of gaseous CsBO will be presented as a keynote speech of the 64th annual conference on Mass Spectrometry. Gaseous cesium metaborate, CsBO
will be presented as a keynote speech of the 64th annual conference on Mass Spectrometry. Gaseous cesium metaborate, CsBO (g), is predicted to be the main cesium vapor species in severe light water reactor accidents according to the thermodynamic analysis, which treats chemical reactions with B
(g), is predicted to be the main cesium vapor species in severe light water reactor accidents according to the thermodynamic analysis, which treats chemical reactions with B C. But quality of the thermodynamic data of CsBO
C. But quality of the thermodynamic data of CsBO (g) used in this thermodynamic analysis is regarded as poor. Thus, Knudsen-effusion mass-spectrometric measurement of CsBO
(g) used in this thermodynamic analysis is regarded as poor. Thus, Knudsen-effusion mass-spectrometric measurement of CsBO was performed to obtain reliable thermodynamic quantities of CsBO
was performed to obtain reliable thermodynamic quantities of CsBO (g). The standard enthalpy values of formation of CsBO
(g). The standard enthalpy values of formation of CsBO (g),
(g), 

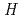
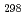
 (CsBO
(CsBO ,g), were obtained by the second and third law treatments and found to be -700.7
,g), were obtained by the second and third law treatments and found to be -700.7 10.7kJ/mol and -697.0
10.7kJ/mol and -697.0 10.6kJ/mol. These two values agree within the error limits and the evaluated thermodynamic data of CsBO
10.6kJ/mol. These two values agree within the error limits and the evaluated thermodynamic data of CsBO (g) are considered to be reliable.
(g) are considered to be reliable.